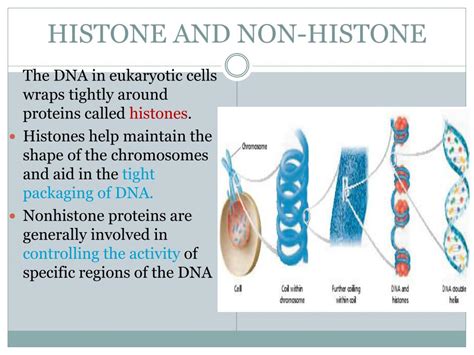
tudor h3 | The histone and non tudor h3 Background: Lamin B receptor (LBR) is an integral nuclear envelope protein and . DusknoirLV. X. Level-Up Pokémon. HP140. Poké-Power. Ectoplasm. If Dusknoir is your Active Pokémon and would be Knocked Out by damage from your opponent’s attack, you may discard all cards attached to Dusknoir LV.Xand put Dusknoir LV.Xas a Stadium card into play instead of discarding it.LOUIS VUITTON Official USA site - Discover our latest Women's Damier Ebene collections, exclusively on louisvuitton.com and in Louis Vuitton Stores.
0 · Tudor: a versatile family of histone methylation ‘readers’
1 · The histone and non
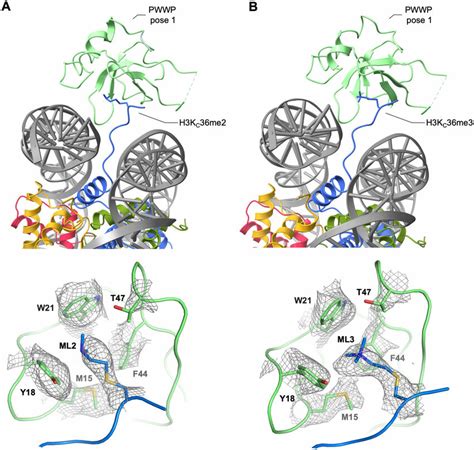
2 · Structure of H3K36
3 · Structural mechanism of bivalent histone H3K4me3K9me3
4 · Recognition of Histone H3 Lysine
5 · Molecular basis for H3K36me3 recognition by the Tudor domain
6 · Distinct mode of methylated lysine
Brady threw for three touchdowns and the Bucs' pass rush stymied Mahomes as the Buccaneers defeated the Chiefs, 31-9, on Sunday in Super Bowl LV at Tampa's Raymond James Stadium.
Here, we discuss novel functions of a number of Tudor-containing proteins (including JMJD2A, 53BP1, SGF29, Spindlin1, UHRF1, PHF1, PHF19 and SHH1) in ‘reading’ unique methylation .Structure of Tudor domains bound to their histone lysine methylation ligands. .National Center for Biotechnology InformationBackground: Lamin B receptor (LBR) is an integral nuclear envelope protein and .
This predicts that Tudor domain binding to the H3K36-methylated nucleosome destabilizes the nucleosome and leads to unwrapping of terminal DNA.Recognition of methylated histone tail lysine residues by tudor domains plays important roles in epigenetic control of gene expression and DNA damage response. Previous studies revealed .
Tudor–histone interaction is critical for regulating various DNA-templated processes. The Tudor domain comprises a family of motifs that mediate protein–protein interactions . In this study, we provide molecular and structural basis by which Spindlin1 acts in complex with C11orf84 to preferentially recognize non-canonical bivalent mark of trimethylated .

Here we show that the Tudor domain of human PHF1 binds to histone H3 trimethylated at Lys36 (H3K36me3). We report a 1.9-Å resolution crystal structure of the Tudor .The Tudor domain comprises a family of motifs that mediate protein–protein interactions required for vari-ous DNA-templated biological processes. Emerging evi-dence demonstrates a .To gain insights into the structural basis for binding methylated histone tails, we have solved the crystal structure of the double tudor domain of JMJD2A both in the presence and absence of a .
The UHRF1 tandem Tudor domain (TTD) binds lysine 9 di- and tri-methylation on histone H3 (H3K9me2/me3) and all three methylation states of lysine 126 on LIG1 (LIG1K126) .We report a 1.9 Å resolution crystal structure of the Tudor domain in complex with H3K36me3 and describe the molecular mechanism of H3K36me3 recognition using NMR analysis. Binding of .TUDOR) failed to associate with H3 when compared with the full-length formofSMN but seemed toberequired for theinteraction because the amino terminus (SMN Nterm) and carboxy terminus (SMN Cterm), which lack the TUDOR domain, only bound weakly to H3 (Fig 2B). Thus, we conclude that the TUDOR domain is required in vitro but not sufficient for .
To screen for combined binding of TTD to H3 peptides with multiple modifications, we used CelluSpotsTM peptide arrays 39, which contain 275 different H3 histone tail peptides with up to four modifications (Figure 1B). Analysis of the results generated a binding specificity profile of TTD to modified H3 peptides which is shown in Figure 1C. As Second Tudor-Like Domain of Spindlin1 Binds the H3K4me3 Peptide. The 2.1 Å structure of human Spindlin1 in complex with a histone H3K4me3 peptide (aa 1–8) shows that it comprises three juxtaposed tudor-like domains (Fig. 1).Each tudor-like domain is composed of four antiparallel β-strands, β1 to β4, whereas the second tudor-like domain (domain II) .
Furthermore, the Tudor domains of SETDB1 bind to native histone H3, but not to unmodified recombinant H3 and there is increased binding to H3 proteins treated with HDAC inhibitors in cells .
Yang, N. et al. Distinct mode of methylated lysine-4 of histone H3 recognition by tandem tudor-like domains of Spindlin1. Proc. Natl Acad. Sci. USA 109 , 17954–17959 (2012).H3 residues are labeled in green and Tudor residues are labeled in brown. Dashed lines represent intermolecular hydrogen bonds. For clarity, here and throughout the text, individual residues of the Tudor domain are denoted using a single-letter code, whereas individual residues of the histone peptide are denoted using a three-letter code. .H3 Tudor Rd Altrincham WA14 5RZ Property For Lease Commercial Real Estate Lancashire Altrincham H3 Tudor Rd, Altrincham, CHS WA14 5RZ. Map Property Overview. The units on Tribune Road form part of the popular and established Hanover Industrial Estate at Altrincham Business Park located off George Richards Way; one of the main employment areas .
Tudor: a versatile family of histone methylation ‘readers’
the Tudor domains and the inter-domain linkers, indicating that 3TD folds and functions as a single unit (Supplementary Fig. 2a). The histone H3 peptide lies in a groove formed by TD2, TD3, and
To test whether other domains of UHRF1 also bind unmodified histone H3, we performed ITC analyses using unmodified H3 titrating the tandem Tudor domain and SRA domain, both of which are important . The PHD finger protein 1 (PHF1) is essential in epigenetic regulation and genome maintenance. Here we show that the Tudor domain of human PHF1 binds to histone H3 trimethylated at Lys36 (H3K36me3). The cocrystal structure of the JMJD2A double tudor domain with a trimethylated H3-K4 peptide reveals that the trimethyl-K4 is bound in a cage of three aromatic residues, two of which are from the tudor-2 motif, whereas the binding specificity is determined by side-chain interactions involving amino acids from the tudor-1 motif. Our study .
The Tudor domain of human PHF1 recognizes trimethylated lysine 36 of histone H3 (H3K36me3). This interaction modulates the methyltransferase activity of the PRC2 complex
Here is where you meet the Ultra and get the platinum graded information. I am HG Tudor. I will provide you with insight and information about me and my kind. I have been doing so for several . After some searching around I came across the H3 dial and now I can't decide which way to go. So I did the only reasonable thing and ordered both dials. . OMEGA, Rolex, Breitling, Rolex and Tudor, Seiko, Grand Seiko and others. Show Less . Full Forum Listing. Explore Our Forums. Public Forum Watches - Private Sellers and Sponsors Affordable .Here, we discuss novel functions of a number of Tudor-containing proteins (including JMJD2A, 53BP1, SGF29, Spindlin1, UHRF1, PHF1, PHF19 and SHH1) in ‘reading’ unique methylation events on histones in order to facilitate DNA damage repair or regulate transcription. This predicts that Tudor domain binding to the H3K36-methylated nucleosome destabilizes the nucleosome and leads to unwrapping of terminal DNA.
Recognition of methylated histone tail lysine residues by tudor domains plays important roles in epigenetic control of gene expression and DNA damage response. Previous studies revealed the binding. Tudor–histone interaction is critical for regulating various DNA-templated processes. The Tudor domain comprises a family of motifs that mediate protein–protein interactions required for various DNA-templated biological processes. Emerging evidence demonstrates a versatility of the Tudor family domains by identifying their specific .
In this study, we provide molecular and structural basis by which Spindlin1 acts in complex with C11orf84 to preferentially recognize non-canonical bivalent mark of trimethylated lysine 4 and.
Here we show that the Tudor domain of human PHF1 binds to histone H3 trimethylated at Lys36 (H3K36me3). We report a 1.9-Å resolution crystal structure of the Tudor domain in complex with.The Tudor domain comprises a family of motifs that mediate protein–protein interactions required for vari-ous DNA-templated biological processes. Emerging evi-dence demonstrates a versatility of the Tudor family domains by identifying their specific interactions to a wide variety of histone methylation marks.
The histone and non
To gain insights into the structural basis for binding methylated histone tails, we have solved the crystal structure of the double tudor domain of JMJD2A both in the presence and absence of a trimethylated H3-K4 peptide (H3K4Me3).
The UHRF1 tandem Tudor domain (TTD) binds lysine 9 di- and tri-methylation on histone H3 (H3K9me2/me3) and all three methylation states of lysine 126 on LIG1 (LIG1K126) . The contribution of this non-histone methyllysine-driven interaction to UHRF1-dependent DNA methylation maintenance is unclear.
Product description. The Duracell Low Voltage Transformer gives you everything you need to create your perfect outdoor lighting system. The low voltage transformer has a maximum power capacity of 23 watts, meaning an incredible 23 lights* can be powered from a single transformer.
tudor h3|The histone and non